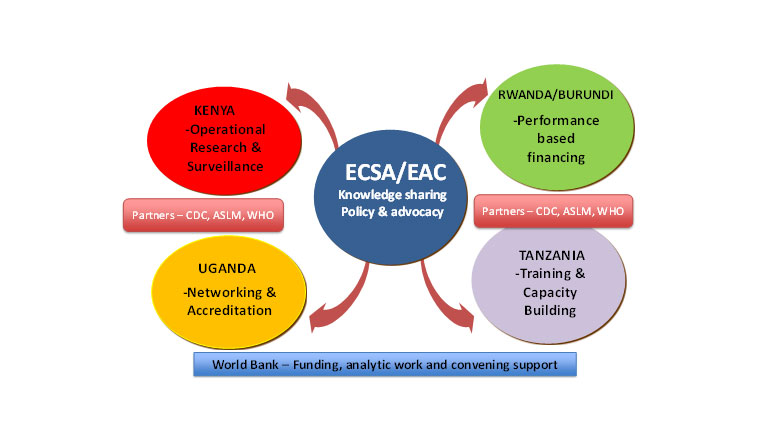
Partners
The project has supported multiple partnerships to leverage additional support.
- Centers for Disease Control and Prevention: conducted scoping missions to inform project design; provided support at country level; partnered with Bank to design the Uganda SRL with construction funded by the Bank.
- WHO: prepared simulations to document TB diagnostic gaps; participated in Mid-Term Review; provided External Quality Assessment panels to national laboratories through National Institute for Communicable Diseases.
- African Society for Laboratory Medicine: trained and certified laboratory assessors; and participated in annual peer assessments.
- Global Fund: provided US$6 million grant to operationalize the Uganda SRL.
Moving Forward
With the recently approved US$50 million Additional Financing the East Africa sub-region will redouble disease prevention and control efforts, drawing lessons from the Ebola outbreak which demonstrated the importance of investing smartly and strategically in disease surveillance and diagnostic systems. The Global Fund support for the Uganda SRL bodes well for sustainability, as regional capacity established under the Bank project will be consolidated and institutionalized. The country-led approach has fostered ownership and capacity building has improved the probability of institutional sustainability. Additional work is needed to ensure financial sustainability of ongoing investments which require substantial recurrent cost financing.
Beneficiaries
“The launching of Gene-Xpert is a major milestone in diagnosis of TB. This is particularly important for us in Wajir where patients travel hundreds of miles to get services.” Medical Officer, Kenya.
“Two months before I visited the Mbale Hospital I had been receiving treatment for fever and flu at a clinic near my home without any sign of recovery. I thought it was HIV”, says Aliyi Mwanika, a 30-year old motorcycle-rider. With the aid of the Gene Xpert machine his illness was correctly diagnosed as Multi-drug resistant TB and he was placed on treatment at the Bank-supported facility. “After six months I was able to go back to work”, notes Mwanika.
“I visited several private clinics in town where laboratory results had shown that I had no TB. I was taking antibiotics without any sign of improvement. I was getting weaker with each passing day”, explains Masaba Enoch, a 37-year old businessman with a persistent cough. With the early introduction of the Gene Xpert machine at the Mbale Regional Referral Hospital he was quickly diagnosed with TB. “After receiving treatment for two weeks, I was informed that I was suffering from the multi-drug resistant TB” he says. “I feel better. I am happier now” Masaba notes after initiating treatment.
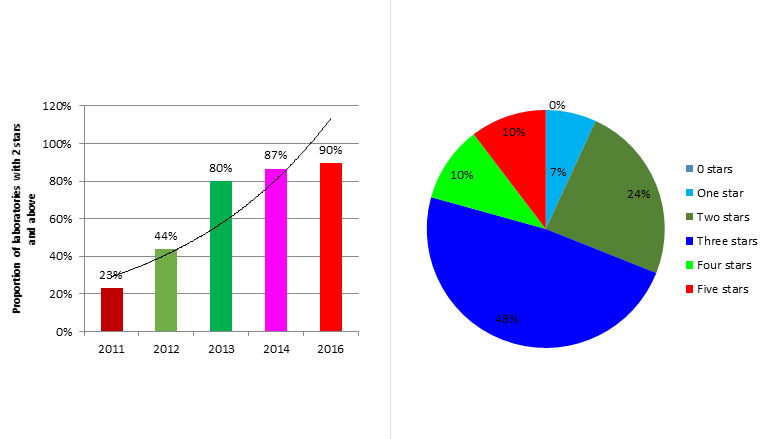
“The accreditation of the Uganda TB laboratory by the reputable South African National Accreditation System is encouraging. It shows how together we can build strong regional institutions capable of serving others laboratories in the network”, Alex Opio, Project Coordinator.
“Financial incentives played a major role in improvements since staff worked harder as they see the fruits of their work through the rise in accreditation status, better managed, safe and equipped labs”, Gilbert Biraro, Rwanda technical expert.